Amino Acid Properties
While working with different enzymes, I always ask myself the question whether a particular amino acid is important for that enzyme’s function or structure and whether a change to that amino acid is damaging to its function? To understand the function of an amino acid, it is useful to know what the different properties of amino acids are and what properties are shared between them. So, here I would like to share with you my cheatsheet which gives a quick and simple overview of the most important amino acids and their properties.
As we know, proteins and peptides are build from various amino acids, which are linked together by the ribosome during a process called translation. There are 22 proteinogenic amino acids out of which 20 are encoded by the standard genetic code. All amino acids have common elements, such as an amine group (N-terminal group in proteins), a carboxyl group (C-terminal group in proteins) and a side chain. The side chain can have various functional groups and gives each amino acid distinct physical properties that influence the final protein, it’s structure and function. There are many groupings available, but in general these groupings are based on the side chain. Here is one of these groupings that I found useful and wanted to share with you.
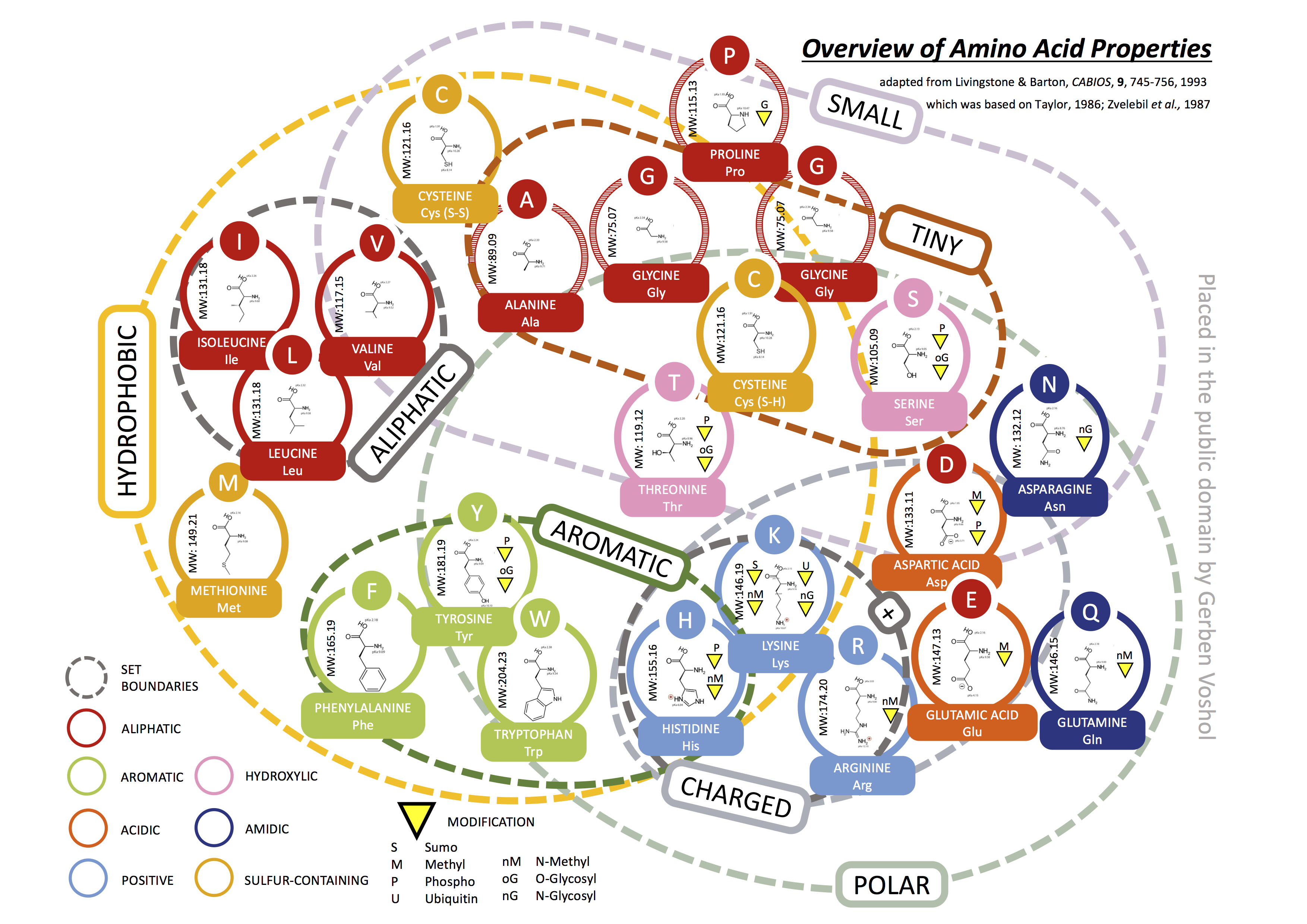
As you might have noticed, glycine is indicated twice in the venn diagram. This is not a mistake, it is just because amino acid glycine is a little bit “strange”. In general, glycine is considered to be a hydrophobic amino acid. Hydrophobic amino acids are usually located inside a protein, but glycine is actually found mainly on the outside of an helix (known as solvent exposed). Moreover, it doesn’t really have a side chain. Therefore it is also placed outside of the hydrophobic group in the diagram. Another “strange” amino acid is proline, which is found often in the loops of proteins where it helps in maintaining a certain fold. Because glycine and proline play such a unique role in protein structure, they are often conserved within protein families. In general, amino acids within the same group are more often subsituted for each other and the amount of set boundaries that one has to cross to get from one amino acid to another is inversely correlated with the likelyhood of such an substitution occurring.